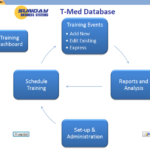
Sunday Business Systems offers two very similar products for employee training management for different levels of compliance. The SBS Training Database and the SBS T-Med Database both offer solutions for defining roles and responsibilities, training requirements, and recording training history, etc. (refer to the Feature Comparison Table). The Training Database was designed for basic compliance where there are slightly less rigorous requirements. The database is an excellent source of objective evidence that training programs have been defined and are being executed in an efficient manner. It is also an invaluable tool to ensure measures of training effectiveness are maintained. Both an Access stand-alone version and a SQL version for enhanced security and performance are available. The SBS T-Med Database was … Continue reading Training and T-Med Databases Compared
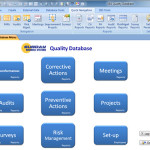
Sunday Business Systems offers two very similar products for different levels of compliance. The SBS Quality Database and the SBS Q-Med Database both offer solutions for Corrective and Preventive Actions (CAPA), Non-conformance management, audit management, etc. (refer to the Feature Comparison Table. The Quality Database was designed for basic compliance where there are less rigorous requirements. The database is an excellent source of objective evidence that, for instance, corrective actions were completed and they were effective. The recorded data is sufficient to show the results and efficacy of the Corrective action program. The SBS Q-Med Database was designed for rigorous compliance to FDA standards (21 CFR Part 820 and ISO 13485) with CFR21 Part 11 compliant electronic records and electronic … Continue reading Tech Note: SBS Quality Database and Q-Med Compared
14-Mar-2016 Sunday Business Systems, a leader in affordable and efficient software tools for quality management systems, is proud to announce the latest release of SBS T-Med Database. This new release is ideal for FDA regulated companies that must manage employee training records. The T-Med Database is an ISO 13485 compliant program used to track employee roles and responsibilities, position training requirements, certification, re-certification, and training events. Simple reports show when re-certification is due or nearly due. Scan and link class rosters, certification checklists, and test results to create an efficient paperless system. The program has a rich set of reports including employee training history for periodic evaluations or reviews. Measure training effectiveness with a click of a button. It utilizes … Continue reading Press Release: T-Med Database Release
KEY FEATURES Nonconformance Tracking Escalate a Nonconformance to a Corrective Action Corrective Actions (CAPA) Safety, Supplier, Internal Audit CARs With Validation and Verification Approvals require electronic signatures Preventive Actions (PA) Audit management Record audit findings Manage Audit Schedules Escalate to a Corrective Action or Preventive Action Meeting management Project management Customer satisfaction surveys CFR21 part 11 compliant security, activity logs, audit trails BENEFITS Complies with requirements of ISO 13485, 21 CFR Part 820, ISO 14971 ISO 9001:2015 AS9100 ISO/TS 16949 Provides a concise, electronic record of historical events, actions, and improvement No paper to get lost Efficiently maintains quality records Internal audits Corrective Action results … Continue reading Q-Med Database