The Best Value In Quality Management Software
Manage compliance for ISO 9001 / 13485 / 14001 / 17025 / 27001 / 45001, AS9100, & IATF 16949

Affordable Performance
Amazing features for the price
Cloud or On-Premise
Choose SaaS or local installation
Scalable Licensing
Single, 3, 5, or unlimited user plans
Modular Flexibility
Pick modules that fit your workflow
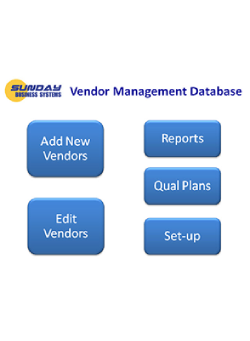
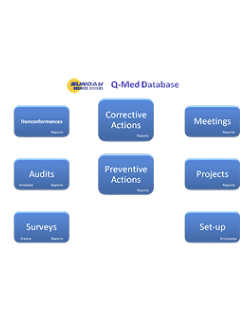
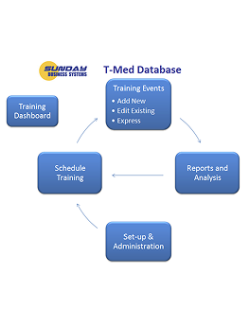
One Integrated Suite. Five Specialized Modules
The SBS QMS Suite manages documents, employee training, CAPA, Nonconformances, audits, risk management, calibration, preventive maintenance, inspection SPC, and supplier management and more. Modules may be purchased separately and are either installed locally or available in the cloud.

Designed for Efficiency, Security, & Compliance
Built to optimize performance, strengthen data security, and sustain compliance with Quality Management System requirements
Password-protected
Access
Passwords and priveleges control access to features
SQL
Backend
Robust SQL architecture or stand-alone option
Cloud or Local
Installation
Choose on-premise or cloud
Scalable
Licensing
Flexible concurrent-user licensing
Modular
Expansion
Add modules as your quality system grows
The Best
Value
Affordable, practical, and powerful
Fast
Deployment
Improve your quality system in hours, not weeks
Easy To learn, Simple
To Use
Intuitive design means minimal training time
Complete Quality Management Suite
The QMS suite may be purchased together or in any combination of modules
Other Products
Built For Regulated Industries
Designed to help organizations meet ISO 9001, AS9100, and ISO 13485 requirements – simplifying compliance, reducing risk, and driving continuous improvement.








What Our Customers Are Saying
Trusted by Quality Professionals Worldwide

It is simple to use; I figured it out with minimal training. Its simplicity makes training our team much easier. It checks all the boxes required by any quality system. Overall, this software is an excellent option for companies seeking affordable QMS solutions. SBS handles all software customizations at a very reasonable price.

Training Database
Thank you for your patience, understanding, hard work and exceptional problem solving skills. You have done truly an exceptional job and we are all so excited to have our new system!

SW (Automotive Industry)
It is easy to use, and to train others to use. We use this on a daily basis for employee training data. Before Sunday Systems software we were using spreadsheets to keep track of employee training. We are a growing company going from around 120 when we began using the software and are currently at 300. The software presents no problems with the added data.

Quality Database
This software is an excellent choice for companies seeking affordable Quality Management System (QMS) solutions. SBS provides all software customizations at a very reasonable price.

BH (Manufacturing)