Sunday Business Systems is proud to announce the latest release of the Quality Database. Version 4.95 improves the user experience, fixes minor bugs, adds checks for data integrity, and adds new NC reports.
KEY FEATURES Nonconformance Tracking and Analysis Closed loop Corrective Actions with Validation and Verification (CAPA) Audit Management Risk Management (FMEA and SWOT Analysis) Continual Improvement Projects Preventive Actions QMS Tools: Context of the Organization, Interested Parties, QMS Scope Meeting Manager Customer Satisfaction Surveys Environmental Health and Safety (EHS / HSE) management email reminders for open CARs, open actions, etc. Rich set of reports and analysis tools for each module Locally installed or Cloud-based solutions BENEFITS Build a simple and efficient Quality Management System (QMS / eQMS) Improve customer satisfaction Quickly and effectively respond to customer complaints Rebuild customer confidence when things go wrong Monitor Customer Perceptions and take action to improve Efficient and cost effective Simple, low cost … Continue reading Quality Database
KEY FEATURES FMEA = Failure Modes and Effects Analysis Risk Assessment: Failure Modes and Effects Analysis (FMEA) Implement risk based strategies for ISO 9001:2015, ISO 14971 Identify failure modes for each process or item Identify effects and severity Identify causes and frequency Identify current controls and detection levels Develop multiple actions associated with this failure mode Assign owners and due dates Establish verification and validation criteria Electronic signature for management approval User login: define user passwords and privileges Rich set of reports Track open actions and delinquent due dates ADVANTAGES Build a simple and efficient Quality Management System (QMS) Install on your local server or leverage our Cloud QMS solution Implement risk based thinking required by ISO 9001:2015 Easy to … Continue reading FMEA Database
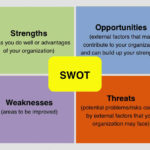
13-Oct-2016 SBS is proud to announce the release of version 4.45 of our Quality Database. This new release includes new SWOT Analysis features. SWOT Analysis analyzes a business’s Strengths, Weaknesses, Opportunities, and Threats, making it ideal for the new requirements of ISO 9001:2015. With FMEA (Failure modes Effects Analysis) used for process/product level risk analysis and SWOT analysis used for business opportunity analysis or competitive analysis, a company can easily demonstrate compliance to the risk management requirements of ISO 9001:2015 and ISO 13485 and complies with ISO 14971. Click here for free demo downloads.
22- Aug-2016 Sunday Business Systems, a leader in affordable and efficient software tools for quality management systems, is proud to announce the first successful audited deployment of the SBS QMS suite in support of ISO 9001:2015. The new ISO 9001:2015 standard has brought several important changes to QMS (Quality Management System) requirements. In particular, eliminating preventive actions and emphasizing risk management has led to considerable consternation and controversy. Sunday Business Systems software is uniquely designed to meet the new requirements of the 2015 standard. SBS consulting services were contracted to begin the process of implementing a fully compliant QMS at an electronics manufacturer in Northern California. The SBS QMS suite was deployed: Asset Tracking, Quality Database, Ground Control, and the … Continue reading SBS QMS Suite: First deployment for ISO 9001:2015
The SBS Quality Database has been upgraded to better meet the requirements of ISO 9001:2015. In addition to the existing features like Corrective Actions, Non-conformance Management, Audit management, etc. we have added a module for Risk Management. Changes to Address Risk and Opportunities The 2015 version of the standard requires the organization promote the use of the risk-based thinking and to determine the risks and opportunities when planning the Quality Management System (QMS). To help ensure good customer satisfaction, the organization must address the risks and opportunities that can affect product or service conformity. The organization must then take actions to address the risks and opportunities and ensure these actions are proportionate to the potential impact. Finally, the organization must … Continue reading The SBS Quality Database for ISO 9001:2015
Sunday Business Systems offers two very similar products for different levels of compliance. The SBS Quality Database and the SBS Q-Med Database both offer solutions for Corrective and Preventive Actions (CAPA), Non-conformance management, audit management, etc. (refer to the Feature Comparison Table. The Quality Database was designed for basic compliance where there are less rigorous requirements. The database is an excellent source of objective evidence that, for instance, corrective actions were completed and they were effective. The recorded data is sufficient to show the results and efficacy of the Corrective action program. The SBS Q-Med Database was designed for rigorous compliance to FDA standards (21 CFR Part 820 and ISO 13485) with CFR21 Part 11 compliant electronic records and electronic … Continue reading Tech Note: SBS Quality Database and Q-Med Compared
KEY FEATURES Implement Risk based strategies for ISO 9001:2015 Identify failure modes for each process or item Identify failure effects and severity Identify causes and frequency Identify current controls and detection levels Develop multiple actions associated with this failure mode Assign owners and due dates Establish verification and validation criteria Electronic signature for management approval Rich set of reports Track open actions and delinquent due dates BENEFITS Save time and reduce the cost of compliance to Quality Standards such as ISO 9001:2015 Implement Risk Based Thinking Easy to use Collaborative platform Simple, menu driven user interface Easy to train users Convenient, intuitive forms Can restrict users to control data integrity Rich set of reports Easy to identify delinquencies, stalled actions … Continue reading Risk Management
Q-MED KEY FEATURES Nonconformance Tracking Corrective Actions (CA) Preventive Actions (PA) Audit management Risk Management – FMEA and SWOT Analysis Meeting management Project management Customer satisfaction surveys CFR21 part 11 compliant security, activity logs, audit trails User login: define user passwords and privileges BENEFITS Complies with requirements of ISO 13485 21 CFR Part 820 ISO 9001:2015 AS9100 ISO/TS 16949 API Q1 Provides a concise, electronic record of historical events, actions, and improvement Efficiently maintains quality records Improve product reliability and process performance Improve Customer Satisfaction Efficient and cost effective Simple Reports and analysis
KEY FEATURES Non-conformance Tracking and Analysis Corrective Actions Supplier Corrective Actions (SCAR) Safety Corrective Actions Preventive Actions Risk Management – FMEA and SWOT Analysis Internal audits Document Management Review Meetings Customer Satisfaction Surveys Email reports directly to employees Define user passwords and privileges BENEFITS A vital software tool to ensure ISO 9001:2015, AS9100, ISO/TS 16949, API Q1 compliance Improve Customer satisfaction Build a simple and efficient Quality Management System (QMS) Paperless QMS Efficient and cost effective
Free Demo Downloads – try before you buy! We believe software must be experienced before you can make a good purchasing decision. Accordingly, we provide free, full-featured demo versions of our standard products that may be downloaded and evaluated prior to purchase. SBS Product Key Features Training Database · Maintain training records · Record certification and re-certifications · Evaluate training effectiveness · Email delinquency notices · Manage training compliance T-Med Database · Ideal for FDA regulated industries · Manage training compliance · Maintain training records · Record certification and re-certifications · Evaluate training effectiveness · Email delinquency notices · CFR 21 Part 11 compliant Quality Database · Manage corrective actions (CARs) · Manage preventive actions (PARs) · Track Non-conformances · Internal … Continue reading Demos
02-May-2018 – SBS is proud to announce the release of version 4.73 of our Quality Database. This new release is designed to improve the user experience by adding automatic tracking number configuration with configurable prefixes, several new Nonconformance reports and report improvements. It also includes performance enhancements and minor bug fixes. 17-Aug-2017 – SBS is proud to announce the release of version 4.57 of our Quality Database. This new release is designed to improve the user experience by adding new buttons to show key forms in datasheet view. It also included performance enhancements and minor bug fixes. 27-Mar-2017 – SBS releases version 4.56 of our Quality Database. This new release includes a new CAR prompt to update Risk Analysis components … Continue reading New Product Release Summary
KEY FEATURES Nonconformance Tracking Escalate a Nonconformance to a Corrective Action Corrective Actions (CAPA) Safety, Supplier, Internal Audit CARs With Validation and Verification Approvals require electronic signatures Preventive Actions (PA) Audit management Record audit findings Manage Audit Schedules Escalate to a Corrective Action or Preventive Action Meeting management Project management Customer satisfaction surveys CFR21 part 11 compliant security, activity logs, audit trails BENEFITS Complies with requirements of ISO 13485, 21 CFR Part 820, ISO 14971 ISO 9001:2015 AS9100 ISO/TS 16949 Provides a concise, electronic record of historical events, actions, and improvement No paper to get lost Efficiently maintains quality records Internal audits Corrective Action results … Continue reading Q-Med Database
ISO 9001 defines the elements of an effective Quality Management System (QMS). A QMS is a set of policies, processes and procedures required for an organization to plan and execute it’s business (production/development/service). SBS Software tools help with efficient compliance to ISO 9001:2015. The Quality Database offers solutions for: Corrective actions Preventive Actions Nonconformance Management Audit Management Risk Analysis (FMEA, SWOT, risk register) Customer perception measurement The Ground Control Database offers solutions for: Document Control Employee Training Management (Training Database also sold separately) The Asset Tracking Database offers solutions for: Calibration Control and recall Preventive Maintenance The Vendor Management Database offers solutions for: Approved Vendor’s List (AVL) Vendor Qualification and Management The SBS Inspection Database offers solutions for: Record incoming, … Continue reading ISO 9001:2015