SBS offers Cloud Based Quality Management Systems (QMS) solutions accessible from any internet enabled device
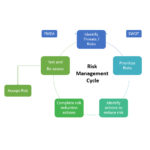
Risk is the possibility of events or activities impeding the achievement of an organization’s strategic and operational objectives. With ISO 9001:2015, the organization must consider the risks and opportunities in optimizing the Quality Management System (QMS) SBS offers several tools to implements Risk assessment in QMS: –The SBS FMEA database A stand-alone tool for analyzing processes or items for failure mechanisms and implementing actions to improve the process and reduce overall risk –The SBS Quality Database Containing tools for Failure Modes and Effects Analysis (FMEA), SWOT analysis, and Preventive Actions. –The Q-Med Database Containing tools for Failure Modes and Effects Analysis (FMEA), SWOT analysis, and Preventive Actions. Ideal for FDA regulated industries
The SBS Asset Tracking Database is the ideal tool: Develop preventive maintenance (PM) schedules for specific equipment Time based PMs Counter based PMs (such as each 10,000 parts run) Rich set of reports showing overdue and upcoming PMs Prevent damage to equipment from “forgotten” maintenance Reduce risk of equipment downtime effecting product Quality and delivery schedules Efficient and cost effective software solution Simple, low cost solution Save time and improve efficiency Show the status of your equipment with a click of a button A vital tool to ensure ISO 9001:2015 compliance Paperless system
Calibration control is critical to ensure product conforms to specific product requirements such as process limits, specifications, or drawings. Your organization is required to provide and maintain the monitoring and measuring devices needed to verify conformity to product requirements. The organization also must retain appropriate documentation as evidence that monitoring and measuring devices are fit for use. The SBS Asset Tracking Database is our premier product for managing monitoring and measuring devices (assets): Control your calibrated equipment Display your recall list Track calibration history Efficient and cost effective software Simple, low cost solution for Calibration Control Saves time and improves efficiency Shows the status of your equipment with a click of a button A vital tool to ensure ISO 9001:2015 … Continue reading Calibration Control
SBS Vendor Management Database is a simple, affordable tool used to manage suppliers or vendors. Establish criteria for selection, evaluation and re-evaluation of suppliers. Classify Vendors or Suppliers using risk based thinking Develop qualification plans Qualify your Suppliers Record qualification results Re-Qualify suppliers as required Maintain your AVL or Approved Vendor List. A rich set of reports highlights qualification gaps and delinquencies. The program is ideal for small businesses striving for ISO 9001 and AS9100 compliance. Click here for more details SBS Vendor Management is a simple, affordable tool used to manage suppliers or vendors. Develop qualification plans, record qualification results, and maintain your AVL or Approved Vendor List. Establish criteria for selection, evaluation and re-evaluation of suppliers. A rich … Continue reading Supplier Management
Quality Management standards require many things including: Monitoring and measurement of processes Monitoring and measuring of products Validation of processes for production and service Design and development verification and validation Control of records An effective Statistical Process Control program will meet these requirements and improve product/process characteristics The SBS Inspection Database was created to ease the complexity of compliance to quality management system standards: ISO 9001 TS 16949 ISO 13485 Feature Overview: Click here for more details.
The Nonconformance Module is a simple and effective tool to comply with quality management standards. Manage Nonconformances Track non-conforming product Assign defect codes and cost Document disposition (Scrap, rework, use as is) Perform Failure Analysis Analyze defects for continual improvement Analyze and report on defect trends defect cost, cost of poor quality The Nonconformance module is available in both the SBS Q-Med Database and the SBS Quality Database. The Q-Med Database is ideal for FDA regulated industries (Medical Device, Pharmaceuticals) The Quality Database is designed for Manufacturing, Aerospace, Service and other industries.
The Audit Module is a simple and effective tool to document and manage audit results. Schedule audits Document audit findings Convert findings into a Corrective Action (CAR) and / or Preventive Action (PAR) Report and analyze results Great for internal and external audits internal QMS audits Product audits Process audits Supplier audits Customer audits The Audit Module is contained within the Quality and Q-Med Databases: The Q-Med Database is ideal for FDA regulated industries (Medical Device, Pharmaceuticals) The Quality Database is designed for Manufacturing, Aerospace, Service and other industries.
SBS Ground Control is an ISO 9001 compliant program used for Document Control and Employee Training Management. Manage the complete document life cycle of any electronic file with electronic signatures and a simple workflow. Reset training requirements for new document revisions. email release notices and approval reminders Great for small companies competing in an ISO 9001 world.
Manage Employee Training: Define employee roles and responsibilities Establish position training requirements, certification, and re-certification Record Instructor-led and self-paced training activity Record measures of training effectiveness (quizzes, tests, ratings, etc.) Simple reports show when re-certification is due or nearly due Reports employee training history for periodic evaluations or reviews Scan and link class rosters, certification checklists, and test results to create an efficient paperless system Measure training compliance with a click of a button Great for small companies striving for QMS compliance SBS offers 2 version of employee training (LMS) software: The SBS T-Med Database is ideal for FDA regulated industries (Medical Device, Pharmaceuticals). The SBS Training Database is designed for Manufacturing, Aerospace, Service and other manufacturing and service industries. … Continue reading Employee Training Management
Corrective and Preventive Actions Follow an 8D problem solving process to fix problems (corrective actions) so they never happen again. Reduce the risk of future problems (preventive actions) by asking “what can go wrong?” and fixing the issue before it harms the organization. Rich set of reports to track and analyze performance drive actions to completion trend CARs and associated cost CAPA (Corrective And Preventive Actions) modules are available in both the SBS Q-Med Database and the SBS Quality Database: The SBS Q-Med Database is ideal for FDA regulated industries (Medical Device, Pharmaceuticals) The SBS Quality Database is designed for Manufacturing, Aerospace, Service and other industries. Click here for a full list of features and a detailed comparison of the … Continue reading Corrective And Preventive Actions