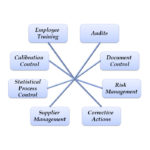
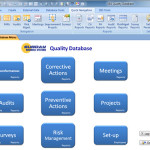
KEY FEATURES Nonconformance Tracking and Analysis Closed loop Corrective Actions with Validation and Verification (CAPA) Audit Management Risk Management (FMEA and SWOT Analysis) Continual Improvement Projects Preventive Actions QMS Tools: Context of the Organization, Interested Parties, QMS Scope Meeting Manager Customer Satisfaction Surveys Environmental Health and Safety (EHS / HSE) management email reminders for open CARs, open actions, etc. Rich set of reports and analysis tools for each module Locally installed or Cloud-based solutions BENEFITS Build a simple and efficient Quality Management System (QMS / eQMS) Improve customer satisfaction Quickly and effectively respond to customer complaints Rebuild customer confidence when things go wrong Monitor Customer Perceptions and take action to improve Efficient and cost effective Simple, low cost … Continue reading Quality Database
KEY FEATURES ISO 9001 compliant vendor management software used to establish criteria for selection, evaluation and re-evaluation of suppliers: Develop Vendor qualification plans Record qualification results Maintain your Approved Vendor List (AVL) Establish criteria for selection, evaluation and re-evaluation of suppliers Create custom supplier qualification plans Maintain a supplier qualification history / supplier performance records Link electronic files (paperless system) Rich set of reports User login: define user passwords and privileges Install on your local server or leverage our Cloud QMS solution BENEFITS Build a simple and efficient Quality Management System (QMS): Comply with standard Purchasing requirements of ISO 9001:2015 AS9100 IATF 16949:2016 ISO 13485 Efficient and cost effective Simple, low cost solution Intuitive design = little training required Saves … Continue reading Vendor Management Database
KEY FEATURES SBS T-Med Database is ISO 13485 compliant software used to track employee training events, position requirements, certification, and re-certification. Report training gaps and key metrics. Manage Employee Training Records Define Job Descriptions / Roles and Responsibilities Define Training requirements by position (standard training for each position) by employee (special training for each employee) for revision controlled documents Certification Requirements Re-Certification Requirements Document revisions trigger training requirements Email Reports directly to affected employees Electronic signature approvals User login: define user passwords and privileges CFR21 Part 11 compliant Install on your local server or leverage our Cloud QMS solution BENEFITS Build a simple and effective Quality Management System (QMS / eQMS) Efficient and cost effective software Simple, low cost solution … Continue reading T-Med Database
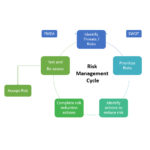
KEY FEATURES FMEA = Failure Modes and Effects Analysis Risk Assessment: Failure Modes and Effects Analysis (FMEA) Implement risk based strategies for ISO 9001:2015, ISO 14971 Identify failure modes for each process or item Identify effects and severity Identify causes and frequency Identify current controls and detection levels Develop multiple actions associated with this failure mode Assign owners and due dates Establish verification and validation criteria Electronic signature for management approval User login: define user passwords and privileges Rich set of reports Track open actions and delinquent due dates ADVANTAGES Build a simple and efficient Quality Management System (QMS) Install on your local server or leverage our Cloud QMS solution Implement risk based thinking required by ISO 9001:2015 Easy to … Continue reading FMEA Database
28-Apr-2017 Sunday Business Systems is proud to announce the latest release of the Asset Tracking Database (version 2.71). This new release is ideal for companies striving to maintain compliance with the ISO 9001:2015 standard. The Asset Tracking Database is an affordable software tool used to manage calibrated equipment, reduce risk through preventive maintenance, and manage unscheduled maintenance. The program has a rich set of reports including calibration recall lists and preventive maintenance schedules. The latest release includes: Added a priority field to the unscheduled maintenance module Improved reports Minor bug fixes and enhancements Free trial downloads are available from our website for a no risk evaluation: https://sundaybizsys.com/asset-tracking-database/
Sunday Business Systems offers two very similar products for employee training management for different levels of compliance. The SBS Training Database and the SBS T-Med Database both offer solutions for defining roles and responsibilities, training requirements, and recording training history, etc. (refer to the Feature Comparison Table). The Training Database was designed for basic compliance where there are slightly less rigorous requirements. The database is an excellent source of objective evidence that training programs have been defined and are being executed in an efficient manner. It is also an invaluable tool to ensure measures of training effectiveness are maintained. Both an Access stand-alone version and a SQL version for enhanced security and performance are available. The SBS T-Med Database was … Continue reading Training and T-Med Databases Compared
Is there an ISO Standard for everything?? ISO 10019:2005 provides guidance for the selection of quality management system consultants and the use of their services. It is intended to assist organizations when selecting a quality management system consultant. It gives guidance on the process for evaluating the competence of a quality management system consultant and provides confidence that the organization’s needs and expectations for the consultant’s services will be met. While the concepts in the standard are sound, interpreting and adapting the standard to meet your individual business needs is critical. Key selection objectives: Ensure the consultant has a proven track record Ensure the plan is focused with achievable objectives Ensure the project time frame is in line with senior … Continue reading Selecting QMS Consultants
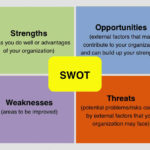
13-Oct-2016 SBS is proud to announce the release of version 4.45 of our Quality Database. This new release includes new SWOT Analysis features. SWOT Analysis analyzes a business’s Strengths, Weaknesses, Opportunities, and Threats, making it ideal for the new requirements of ISO 9001:2015. With FMEA (Failure modes Effects Analysis) used for process/product level risk analysis and SWOT analysis used for business opportunity analysis or competitive analysis, a company can easily demonstrate compliance to the risk management requirements of ISO 9001:2015 and ISO 13485 and complies with ISO 14971. Click here for free demo downloads.
22-Sep-2016 SBS is proud to announce the release of version 1.10 of our FMEA Database. This new release includes a new user interface that improves the look and feel of the software. The release also includes new SWOT Analysis. SWOT Analysis analyzes a business’s Strengths, Weaknesses, Opportunities, and Threats, making it ideal for the new requirements of ISO 9001:2015. With FMEA (Failure modes Effects Analysis) used for process/product level risk analysis and SWOT analysis used for business opportunity analysis or competitive analysis, a company can easily demonstrate compliance to the risk management requirements of ISO 9001 and 13485. See our website for free demo downloads.
Sunday Business Systems offers two very similar products for different levels of compliance. The SBS Quality Database and the SBS Q-Med Database both offer solutions for Corrective and Preventive Actions (CAPA), Non-conformance management, audit management, etc. (refer to the Feature Comparison Table. The Quality Database was designed for basic compliance where there are less rigorous requirements. The database is an excellent source of objective evidence that, for instance, corrective actions were completed and they were effective. The recorded data is sufficient to show the results and efficacy of the Corrective action program. The SBS Q-Med Database was designed for rigorous compliance to FDA standards (21 CFR Part 820 and ISO 13485) with CFR21 Part 11 compliant electronic records and electronic … Continue reading Tech Note: SBS Quality Database and Q-Med Compared
14-Mar-2016 Sunday Business Systems, a leader in affordable and efficient software tools for quality management systems, is proud to announce the latest release of SBS T-Med Database. This new release is ideal for FDA regulated companies that must manage employee training records. The T-Med Database is an ISO 13485 compliant program used to track employee roles and responsibilities, position training requirements, certification, re-certification, and training events. Simple reports show when re-certification is due or nearly due. Scan and link class rosters, certification checklists, and test results to create an efficient paperless system. The program has a rich set of reports including employee training history for periodic evaluations or reviews. Measure training effectiveness with a click of a button. It utilizes … Continue reading Press Release: T-Med Database Release
There have been some important updates to the Asset Tracking Database. 1. We have a new user interface: The new interface is very simple and intuitive, allowing users to move between modules with fewer clicks. 2. We have automatically re-scheduled PMs and Calibrations. 3. We also have the ability to collect parametric data for each calibration: Calibration data collection is great for devices, such as calipers or gauge blocks, that are calibrated internally. Techs can record the actual measurements, compare them to specification limits, and create a quality record for each calibration event. There is no charge for the latest version of the software. However, we charge $95 to import data from your current database into the new database. The … Continue reading What’s new in the Asset Tracking Database
SBS seeks qualified individuals and organizations that understand the requirements and nuances of quality management standards such as ISO 9001, AS 9100, ISO 13485, etc. We offer an opportunity to resell our products to provide financial rewards and to enhance value of the service you provide. BECOME AN AFFILIATE AND… Provide affordable and effective tools to your Customers Expand your Customer relationship Earn a commission on every sale Get client referrals from Sunday Business Systems Add your website to our Links page Provide direct feedback to Sunday Business Systems Gain access to training materials and flow charts PROGRAM DETAILS Register as an approved affiliate (or re-seller) with MyCommerce Share-it (a Digital River Company). Read and agree to the terms and … Continue reading Re-seller Program
International Standards bring technological, economic and societal benefits. They help to harmonize technical specifications of products and services making industry more efficient and breaking down barriers to international trade. Conformity to International Standards helps reassure consumers that products are safe, efficient and good for the environment. For business International Standards are strategic tools and guidelines to help companies tackle some of the most demanding challenges of modern business. They ensure that business operations are as efficient as possible, increase productivity and help companies access new markets. Benefits include: Cost savings – International Standards help optimize operations and therefore improve the bottom line Enhanced customer satisfaction – International Standards help improve quality, enhance customer satisfaction and increase sales Access to new … Continue reading Benefits of International Standards
KEY FEATURES Implement Risk based strategies for ISO 9001:2015 Identify failure modes for each process or item Identify failure effects and severity Identify causes and frequency Identify current controls and detection levels Develop multiple actions associated with this failure mode Assign owners and due dates Establish verification and validation criteria Electronic signature for management approval Rich set of reports Track open actions and delinquent due dates BENEFITS Save time and reduce the cost of compliance to Quality Standards such as ISO 9001:2015 Implement Risk Based Thinking Easy to use Collaborative platform Simple, menu driven user interface Easy to train users Convenient, intuitive forms Can restrict users to control data integrity Rich set of reports Easy to identify delinquencies, stalled actions … Continue reading Risk Management
KEY FEATURES Control of Calibrated Equipment Recall Report for “Out of Cal” Equipment Preventive Maintenance Schedules Track Unscheduled Maintenance Generate Fix-It-Tickets Control Miscellaneous Assets User login: define user passwords and privileges BENEFITS A Vital Tool to Ensure ISO 9001:2015 Compliance Build a simple and efficient Quality Management System (QMS) Efficient and Cost Effective Paperless System
DOCUMENT CONTROL & EMPLOYEE TRAINING MANAGEMENT Complete document life cycle management Controls any electronic document including Microsoft office files: Word, Excel, PowerPoint, PDF, AutoCAD, SolidWorks Control document releases Ensure approvals are documented Organize Electronic Documents New releases reset training requirements Simple, effective reports Measure the effectiveness of your document control system Manage Training Records Define Job Descriptions / Roles and Responsibilities Define Training requirements Manage a Training Schedule Certification Requirements Re-Certification Requirements Document revisions trigger training requirements Email Reports directly to affected employees BENEFITS A vital tool to ensure ISO 9001:2015, AS9100, and ISO/TS 16949, API Q1 compliance Efficient and cost effective Simple document life cycle management Build a simple and efficient Quality Management System (QMS)
Q-MED KEY FEATURES Nonconformance Tracking Corrective Actions (CA) Preventive Actions (PA) Audit management Risk Management – FMEA and SWOT Analysis Meeting management Project management Customer satisfaction surveys CFR21 part 11 compliant security, activity logs, audit trails User login: define user passwords and privileges BENEFITS Complies with requirements of ISO 13485 21 CFR Part 820 ISO 9001:2015 AS9100 ISO/TS 16949 API Q1 Provides a concise, electronic record of historical events, actions, and improvement Efficiently maintains quality records Improve product reliability and process performance Improve Customer Satisfaction Efficient and cost effective Simple Reports and analysis
KEY FEATURES Non-conformance Tracking and Analysis Corrective Actions Supplier Corrective Actions (SCAR) Safety Corrective Actions Preventive Actions Risk Management – FMEA and SWOT Analysis Internal audits Document Management Review Meetings Customer Satisfaction Surveys Email reports directly to employees Define user passwords and privileges BENEFITS A vital software tool to ensure ISO 9001:2015, AS9100, ISO/TS 16949, API Q1 compliance Improve Customer satisfaction Build a simple and efficient Quality Management System (QMS) Paperless QMS Efficient and cost effective
KEY FEATURES Real-time Statistical Process Control (SPC) Configure Collect data for parts and/or processes Define limits: valid data, specification, and control Chart Display options Collect and validate data against Control Limits Spec Limits Correlate data to process, part #, serial number or work order Real-time SPC Control Charts – generates warnings for spec / control limit violations Simple reports show summary statistics including Cp, Cpk Export raw data to Excel, Word, etc. Unlimited users, no per user licensing Ideal for Final QC data entry BENEFITS Efficient and cost effective Simple, low cost solution Saves time and improves efficiency Eliminates paper records A vital tool to ensure ISO9001 compliance Implements basic data collection and validation to ensure product compliance Eliminates paper … Continue reading SPC Keeper