Risk Management
Risk is the possibility of events or activities impeding the achievement of an organization’s strategic and operational objectives.
Quality management standards like ISO 9001, require your organization to consider risks and opportunities in optimizing the Quality Management System (QMS).
SBS offers several tools to implements risk assessments:
The SBS Quality Database
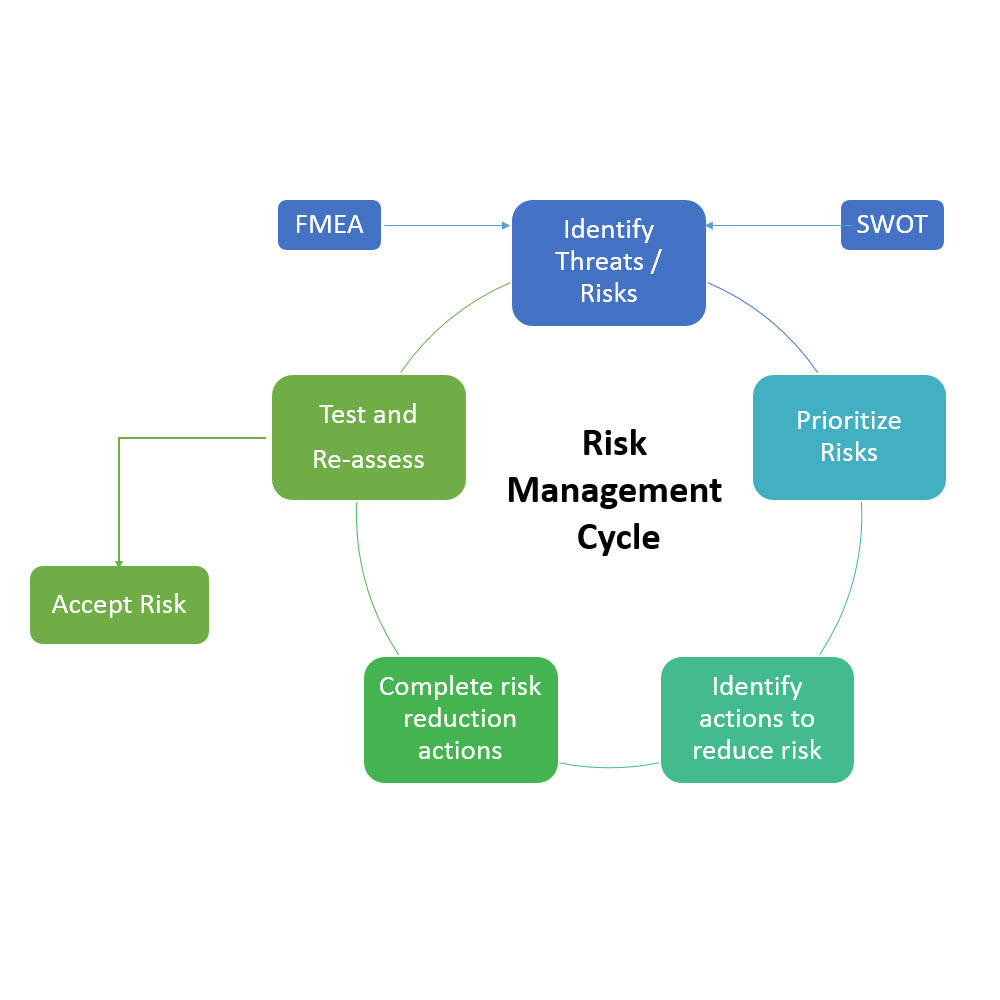
- Utilizes industry standard Failure Modes and Effects Analysis (FMEA)
- SWOT analysis (Strengths, Weaknesses, Opportunities, and Threats)
- Job Hazard Assessments (in the EHS module)
- Risk register detailing risks and opportunities for interested parties
The SBS Q-Med Database
- Containing tools for Failure Modes and Effects Analysis (FMEA), SWOT analysis, and Preventive Actions.
- Ideal for FDA-regulated industries
The SBS FMEA Database
- A stand-alone tool for analyzing product designs and processes for failure mechanisms and implementing actions to improve the design/process and reduce overall risk
- SWOT analysis (Strengths, Weaknesses, Opportunities, and Threats)
- Utilizes Failure Modes and Effects Analysis (FMEA) to analyze process or product-based risk
- Great tool for DFMEAs (Design FMEA) and PFMEAs (Process FMEA)