Sunday Business Systems is proud to announce the latest release of the Quality Database. Version 4.95 improves the user experience, fixes minor bugs, adds checks for data integrity, and adds new NC reports.
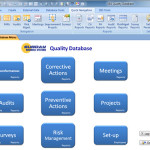
The SBS Quality Database has been upgraded to better meet the requirements of ISO 9001:2015. In addition to the existing features like Corrective Actions, Non-conformance Management, Audit management, etc. we have added a module for Risk Management. Changes to Address Risk and Opportunities The 2015 version of the standard requires the organization promote the use of the risk-based thinking and to determine the risks and opportunities when planning the Quality Management System (QMS). To help ensure good customer satisfaction, the organization must address the risks and opportunities that can affect product or service conformity. The organization must then take actions to address the risks and opportunities and ensure these actions are proportionate to the potential impact. Finally, the organization must … Continue reading The SBS Quality Database for ISO 9001:2015
Sunday Business Systems offers two very similar products for different levels of compliance. The SBS Quality Database and the SBS Q-Med Database both offer solutions for Corrective and Preventive Actions (CAPA), Non-conformance management, audit management, etc. (refer to the Feature Comparison Table. The Quality Database was designed for basic compliance where there are less rigorous requirements. The database is an excellent source of objective evidence that, for instance, corrective actions were completed and they were effective. The recorded data is sufficient to show the results and efficacy of the Corrective action program. The SBS Q-Med Database was designed for rigorous compliance to FDA standards (21 CFR Part 820 and ISO 13485) with CFR21 Part 11 compliant electronic records and electronic … Continue reading Tech Note: SBS Quality Database and Q-Med Compared
Corrective and Preventive Actions Follow an 8D problem solving process to fix problems (corrective actions) so they never happen again. Reduce the risk of future problems (preventive actions) by asking “what can go wrong?” and fixing the issue before it harms the organization. Rich set of reports to track and analyze performance drive actions to completion trend CARs and associated cost CAPA (Corrective And Preventive Actions) modules are available in both the SBS Q-Med Database and the SBS Quality Database: The SBS Q-Med Database is ideal for FDA regulated industries (Medical Device, Pharmaceuticals) The SBS Quality Database is designed for Manufacturing, Aerospace, Service and other industries. Click here for a full list of features and a detailed comparison of the … Continue reading Corrective And Preventive Actions