The Q-Med Database is an ISO 13485 compliant database to efficiently manage corrective and preventive actions, nonconformances, internal audit schedules and findings, safety improvement, supplier corrective actions, continual improvement projects. Compile and analyze Customer Survey results. Document QMS review meetings and results. Cloud based or locally installed options are available. The Q-Med Database is ideal for small businesses striving for ISO 13485 compliance.
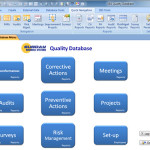
The SBS Quality Database has been upgraded to better meet the requirements of ISO 9001:2015. In addition to the existing features like Corrective Actions, Non-conformance Management, Audit management, etc. we have added a module for Risk Management. Changes to Address Risk and Opportunities The 2015 version of the standard requires the organization promote the use of the risk-based thinking and to determine the risks and opportunities when planning the Quality Management System (QMS). To help ensure good customer satisfaction, the organization must address the risks and opportunities that can affect product or service conformity. The organization must then take actions to address the risks and opportunities and ensure these actions are proportionate to the potential impact. Finally, the organization must … Continue reading The SBS Quality Database for ISO 9001:2015
KEY FEATURES Non-conformance Tracking and Analysis Corrective Actions Supplier Corrective Actions (SCAR) Safety Corrective Actions Preventive Actions Risk Management – FMEA and SWOT Analysis Internal audits Document Management Review Meetings Customer Satisfaction Surveys Email reports directly to employees Define user passwords and privileges BENEFITS A vital software tool to ensure ISO 9001:2015, AS9100, ISO/TS 16949, API Q1 compliance Improve Customer satisfaction Build a simple and efficient Quality Management System (QMS) Paperless QMS Efficient and cost effective